[ad_1]
Earlier this year, the Food & Drug Administration disclosed that it would not regulate non-pharmaceutical CBD products, thereby putting the onus on Congress to devise an appropriate regulatory framework for cannabidiol and other hemp-derived cannabinoids. Solicited by a formal Congressional Request for Information (RFI) on ideas for how to regulate hemp-derived CBD, public feedback included a diverse range of perspectives from businesses, trade associations, and other stakeholders. But today’s “hemp” market has moved way beyond CBD, as noted by several commentators who expressed concerns about the unregulated proliferation of high-dose THC consumables and novel synthetic intoxicants thanks to loopholes in the 2018 Farm Bill, which is up for revision and renewal in the coming months. What follows are comments recently submitted by Tiffany Devitt, a longtime Project CBD contributor, in response to the Congressional RFI on a potential regulatory pathway for hemp-derived CBD. A leading California cannabis industry policy influencer, Devitt is currently Director of Regulatory Affairs at CannaCraft & March and Ash.
Current Market Dynamics
Loopholes in the 2018 Farm Bill
The 2018 Farm Bill defines “hemp” as “the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.”
Within that definition, are two critical phrases: “all derivatives” and “whether growing or not.”
- “All derivatives” is currently being misinterpreted to encompass any compound that can theoretically be chemically synthesized from CBD, even wholly novel compounds not found in the plant in commercially meaningful quantities (if at all).
- “Whether growing or not” is being misconstrued to mean that the 0.3 percent THC limit applies to the plant biomass and the final product, resulting in THC levels in consumer products labeled as “hemp” that substantially exceed THC limits set in state-regulated cannabis markets.
- Lastly, the omission of THCA, a natural compound that converts to THC when heated, is causing further confusion.
Collectively, this language has fostered a regulatory gap that companies are taking advantage of to sell highly intoxicating products, colloquially and misleadingly referred to in the national unregulated hemp marketplace as “legal cannabis.”1
The “Hemp” Market Has Moved Way Past CBD
In the federal discussion about hemp, “hemp” and “CBD” are often conflated. The reality is that the commercial market has largely shifted away from non-intoxicating CBD wellness products to highly intoxicating recreational products. Pseudo-hemp companies with brand names like Fuked Up2 and Clusterf*ck3 make no pretense of selling nutritional supplements. Instead, they market THC-like products that are far stronger than anything found in state-regulated cannabis markets, where the maximum dose of THC per serving is commonly capped at five to 10 milligrams.4 In the unregulated “hemp” market, brands like Chapo Extrax sell products with hundreds of milligrams of synthetic THC per serving.5 The so-called “hemp” market is no longer predominantly a wellness market. It is, in the words of Chapo Extrax, “the newest drug cartel in town.”6
Synthetic Compounds & Escalating Potencies
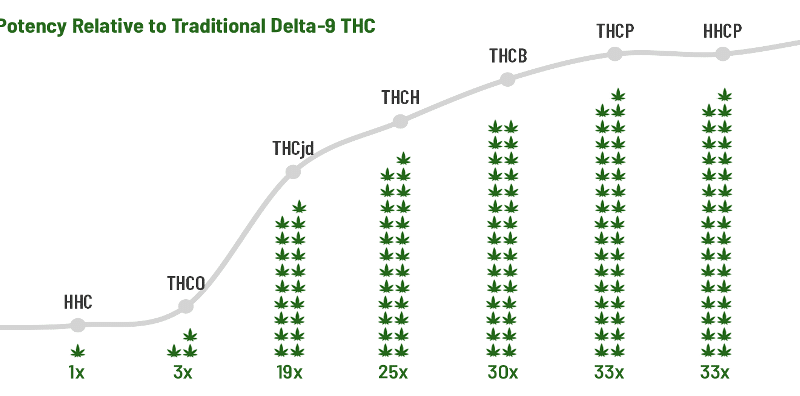
While the recreational hemp market started with delta-8 THC, we’re now seeing pseudo-hemp companies in an all-out race to synthesize new compounds that are significantly stronger than anything naturally occurring. In this respect, the so-called hemp market has much in common with the illicit opiate market. This trend has given rise to a new generation of designer drugs with astonishingly high potencies. For example, one of the most recent synthetic cannabinoids to come to market, “delta-9P,” is 35 times more potent than the natural THC found in cannabis.7

The argument that anything that can be synthesized from hemp-derived CBD is legal is flat-out wrong. Methamphetamines can be synthesized from over-the-counter cough medicine, but that doesn’t make meth legal. In a shocking sign of just how far hemp purveyors are willing to take this thinking, the CEO of the hemp company 3Chi recently asserted in a legal proceeding that if heroin could be synthesized from CBD, it would be exempt from the Controlled Substances Act.8
Safety Issues Associated with Synthetic Cannabinoids
This new generation of designer drugs is reminiscent of “Spice,” “K2,” and other synthetic cannabinoids that emerged in the illicit drug market in the early 2000s.9 We cannot assume that these novel compounds are safe based on the safety profile of natural cannabinoids. On the contrary, there is significant evidence that synthetic cannabinoids are dangerous.10 They have been linked to seizures,11 acute respiratory failure,12 heart attack,13 stroke,14 lung injury,15 kidney damage,16 psychosis,17 and even death.18
Many “Hemp” Cannabinoids Are Made in a Lab – Not from Hemp or CBD
Synthesizing new compounds from hemp-derived CBD requires an enormous amount of biomass. Thus, it’s reasonable to assume that, as the “hemp” product market is exploding, the agricultural market should also be booming. But the opposite is true. The amount of hemp under cultivation in the U.S. has shrunk by 48 percent since 2021.19 This is in part because most of the new compounds being sold as hemp simply cannot be synthesized from CBD.20 They are “man-made chemicals produced in underground labs, often in China, and then shipped to the United States in powder or crystal form.”21
Manufacturing Impurities & Byproducts
Equally important, in the stampede to market “legal cannabis,” so-called hemp companies are willfully ignoring safety issues related to the acid-catalyzed conversion of CBD into THC-like compounds. According to experts, the conversion process can produce numerous additional THC isomers with unknown pharmacological and safety effects.22 These non-natural THC-like isomers are difficult to measure and almost impossible to remove from the end product.23 Yet this conversion is occurring without regulatory oversight to ensure process standardization, product specification, and accurate third-party testing, all of which are mandated in state-licensed cannabis programs.
Marketing to Minors
Unlike state-regulated cannabis, this new wave of acutely intoxicating substances is easily accessible to minors. These products are sold online, in convenience stores, gas stations, smoke shops, and vending machines across the country. A recent study in the Journal of Cannabis Research revealed that around 85 percent of intoxicating hemp brands lack substantial age verification at checkout.24 Over 80 percent did not have child-resistant packaging.25 Additionally, these brands often use child-friendly marketing strategies, such as cartoons and mimicry of popular candies and snacks, practices prohibited in most regulated cannabis markets. Some actively advertise their “discreet” shipping with no adult signature required.26
| Capt’n Chronic27 | Delta-8 Oreos28 | Jolly Rancher29 | Skittles30 |

|

|

|

|
Notably, since Congress passed the 2018 Farm Bill, there has been a significant increase in reported cannabinoid poisonings among children and teens, an uptick that parallels the proliferation of intoxicating “hemp” products.31 The Centers for Disease Control and Prevention (CDC) reports that cannabinoid-related emergency department visits among young people also increased during this period.32
Pathways
The hemp framework under consideration today must prioritize consumer safety, provide a stable agricultural market for hemp farmers, and respect states’ rights to regulate intoxicating cannabinoid products. To achieve this, two things need to happen. First, Congress needs to close the loopholes in the Farm Bill, which was never intended to open the floodgates for intoxicating, synthetic products. Second, appropriate regulatory pathways (under FDA authority) must be defined for three distinct classes of cannabinoid products:
- Non-intoxicating naturally derived cannabinoid products
- Intoxicating naturally derived cannabinoid products
- Synthetic designer drugs
Step 1: Fix the Farm Bill
Based on the realities of the CBD/hemp market described above, it is imperative that the definition of hemp in the Farm Bill be amended to:
- Include THC-equivalent compounds within the THC threshold.
- Clarify that the THC percentage threshold applies strictly to hemp biomass, not finished goods.
- Explicitly exclude novel synthetic compounds from the hemp definition and subject them to FDA oversight.
Step 2: Regulatory Pathways
Considering the public health crisis posed by the proliferation of intoxicating synthetic drugs sold as hemp, we believe that Congress and the FDA must utilize existing pathways to regulate hemp-derived cannabinoid products under FDA and state authorities, as described below. Concurrently, Congress must work with the FDA to develop a much-needed regulatory framework for the unique cannabinoid marketplace.
- Non-Intoxicating Naturally Derived Cannabinoid Products
This category includes non-intoxicating natural cannabinoids that can be extracted from hemp (or cannabis), such as CBD, CBDA, and CBG. These compounds should be authorized as food additives consistent with state law and regulated appropriately under federal and state authorities based on the product’s end use, i.e., topicals, ingestibles, and inhalables. To mitigate toxicological concerns, the FDA might consider including a warning about the importance of consulting with one’s doctor on potential CBD-drug interactions. (It is worth noting that CBD is not the first natural compound to be used as an ingredient in both prescription drugs and foods, cosmetics, and over-the-counter medications. Caffeine can also be found in pharmaceutical33 and non-pharmaceutical products.) - Intoxicating Naturally Derived Cannabinoid Products
This category includes traditional delta-9 THC and other naturally occurring and intoxicating cannabinoids. These compounds (including delta-8) are molecularly similar to THC and arguably are THC analogues, as defined in the Federal Analogue Act, 21 U.S.C. § 813. As such, they should be treated in a manner that aligns with state-regulated cannabis products. All intoxicating cannabinoid products – whether derived from cannabis or hemp (which are the same plant) – should fall under the jurisdiction of state cannabis programs and be subject to the same sales and marketing restrictions and safety standards. - Synthetic Designer Drugs
This category includes cannabinoids that are chemically synthesized from hemp or other materials. They are not found in the plant in commercially meaningful quantities (if at all). As these compounds are new and distinct from natural plant cannabinoids, they should be subject to FDA oversight and undergo toxicology studies. This category includes THCO, THCB, THCP, delta9P, THCX, THCH, THCjd, HHCO, HHCP, HXCP, and numerous other new compounds being brought to market at an alarming rate.
In summary, we urge Congress to immediately rectify the loopholes in the Farm Bill and employ established regulatory structures to enable the sale of non-intoxicating CBD products as health supplements – not as starter material for concocting unregulated THC analogues and synthetics.
Safety
The Problem with Percentages
As mentioned earlier, the 0.3 percent delta-9 THC threshold for hemp is currently being misapplied to manufactured products. This is especially troubling in the edible market, where other non-hemp ingredients are present. THC is a potent compound – a dose is typically measured in milligrams (thousandths of a gram), not grams.
As shown in the table below, applying the THC percentage threshold to the final product means that hemp-infused edibles and beverages could have 100 times the amount of THC allowed in most state-regulated cannabis products.
| PRODUCT TYPE | 2 GUMMIES | 1 COOKIE | 1 BROWNIE | 1 BEVERAGE |
| UNIT WEIGHT | 5.8g | 16g | 70g | 340g |
| ALLOWABLE THC (0.3%) | 17.4mg | 48mg | 210mg | 1020mg |
(Unsurprisingly, a recent study found that 26.5 percent of so-called hemp delta-9 products are illegally sourced from marijuana plants.)34
The Need for THC Caps in Hemp Products
Since using a percentage threshold is not a workable regulatory strategy, hemp products must have THC caps to ensure that routine use of these products does not cause intoxication. Moreover, we must bear in mind that how product makers define a serving size often differs from how consumers define it. (Ben & Jerry’s can claim that a single serving of ice cream is just over a half cup, but many of us are eating a lot more.) Thus, the federal standard must specify the maximum THC per serving and package. A typical sub-intoxicating dose of THC for most people is between 0.5 and 2.5mg, depending on the person’s THC tolerance.35 Accordingly, several states36 have adopted the standard of 0.5mg of THC per serving and 2.5mg per package for hemp products. Others, like Washington state, restrict products with any THC to the state cannabis market.
Does CBD Really Have a “THC-Sparing” Effect?
Some hemp marketers have misleadingly argued that CBD has a “THC-sparing” effect. The implication is that CBD neutralizes or counteracts the intoxicating effect of THC,37 and therefore, THC limits can be higher if the two compounds are combined. Not so, according to a recent study in Neuropsychopharmacology that assessed the psychotropic effects of co-administering 10 mg of THC with 0, 10, 20, and 30 mg of CBD. The conclusion: “CBD did not impact THC-induced cognitive impairment, psychotic-like experiences, or ratings of intoxication, anxiety, or drug liking at any dose. These findings do not support the hypothesis that CBD has THC-sparing effects when co-administered according to commonly used doses and routes of administration.”38,39 Other studies suggest that low doses of CBD can amplify THC’s intoxicating effects, while very high amounts (400 or more milligrams) may reduce the THC high somewhat but extend its duration.40 In other words, the quantity of CBD in a hemp product is irrelevant to the question of how much THC is appropriate.
What About THCA?
Another emerging category in the unregulated hemp market is “smokable hemp” or “THCA flower.” THCA hemp flower has high concentrations of THCA and less than 0.3 percent delta-9 THC. However, when THCA is heated – as when smoked – almost 90 percent41 of it converts to traditional THC. In other words, THCA flower is essentially old-fashioned weed that’s been rebranded. Its existence rests entirely on the spurious argument that the Farm Bill doesn’t explicitly mention THCA. Here is a comparison of “hemp THCA flower” and cannabis flower from Cannabis Business Law:42
| “Hemp THCA Flower” | Cannabis Flower | |
| THCA | 25% | 24% |
| Delta-9 THC | 0.18% | 1.24% |
| Total THC when heated | 22% | 22% |
There is no meaningful difference.
It should be self-evident that the total amount of THC in hemp products must account for all potentially intoxicating cannabinoids, including THCA. Indeed, in state cannabis markets, THCA is always included in the definition of THC and the calculation of total THC.
Federal-State Interaction
Mixed Messages & Enforcement Failures
The sale and proliferation of pseudo-hemp designer drugs and “THCA hemp flower” have gone virtually unchecked. Some states have banned these substances, but enforcement has generally been anemic and unsuccessful in the face of an onslaught of online, direct-to-consumer mail-order businesses.
Moreover, several states have been stymied by the conflicting messages from the federal government. The Federal Analogue Act states that any substance that “has a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in Schedule I or II” must be treated like a Schedule I substance.43 That would imply that intoxicating THC analogues synthesized from hemp warrant the same restrictions as marijuana. But in May 2022, the Ninth Circuit Court of Appeals upended the Federal Analogue Act when it ruled that products containing delta-8 THC are lawful under the Farm Bill because they meet the statutory definition of industrial hemp – even though they can get consumers high.44
This contradictory and controversial decision has been cited in several lawsuits45 aimed at overturning state laws restricting the sales of intoxicating products that product-makers claim to be derived from hemp. A Kentucky court referenced the Ninth Circuit’s decision when it rejected an argument that delta-8 is a derivative of a derivative and is, therefore, synthetic and illegal under the Farm Bill. A Georgia state court46 and the Minnesota legislature47 recently enacted similar positions.
Conclusions & Recommendations
The 2018 Farm Bill was enacted to legalize wildly popular, non-intoxicating CBD products. Instead, it inadvertently opened a Pandora’s Box of high-potency edibles, sketchy vape products, and the cannabis equivalent of bathtub gin. These products are rife with contaminants and chemical byproducts,48 easily accessible and sold without age gates,49 testing standards, packaging and labeling requirements, marketing limitations, or even a proper understanding of their potential effects on consumers. Otherwise put: In 2018, Congress set out to legalize the equivalent of kombucha (CBD) and accidentally appeared to legalize moonshine instead – all the while maintaining the prohibition on traditional THC, the wine or beer equivalent in this analogy.
Four things are needed to rectify this situation:
- The Farm Bill needs to be amended to close the unintended loopholes. Specifically, Congress needs to clarify that (i) the 0.3 percent THC threshold applies only to hemp biomass, not finished goods; (ii) “THC” legally refers to all forms of tetrahydrocannabinol, including THCA, delta-8 THC, and other natural THC analogs; and (iii) novel synthetic compounds are excluded from the definition of hemp and subject to FDA oversight.
- Until cannabis is legalized at a federal level, and to avoid undermining states’ rights to regulate intoxicating cannabinoid products, Congress must apply a cap on the amount of THC and THC-equivalent compounds in hemp consumer products to ensure they are non-intoxicating.
- A pathway needs to be established to sell CBD and other natural non-intoxicating cannabinoids as food additives and nutritional supplements.
- There must be a concerted effort by the FDA and DEA to address the public health crisis spawned by the proliferation of unregulated designer drugs being sold under the guise of hemp.
Lastly, in the long term, Congress should lay the groundwork for a unified regulatory framework for intoxicating cannabinoids, irrespective of whether they’re source from cannabis or hemp.
Tiffany Devitt heads up regulatory affairs for CannaCraft and March and Ash and sits on the board of the California Cannabis Industry Association (CCIA). May not be reprinted without permission.
Footnotes
- https://www.instagram.com/p/CszDtayPScA/?utm_source=ig_web_copy_link&igshid=MzRlODBiNWFlZA==
- https://fukedup.com/
- https://hionnature.com/products/3000mg-knockout-gummy-bites-clusterf-cks
- https://www.marijuanaventure.com/edibles-regulations-vary/
- https://chapoextrax.com/product/very-berry-live-resin-3500mg-gummies/
- https://chapoextrax.com/
- https://hightimes.com/sponsored/what-is-delta-9p-everything-you-need-to-know/
- 3C, LLC d/b/a/ 3Chi and MIDWEST HEMP COUNCIL, Plaintiffs, v. ATTORNEY GENERAL TODD ROKITA, in his official capacity, and STATE OF INDIANA, Case No. 1:23-cv-01115-JRS-MKK, U.S. DISTRICT COURT SOUTHERN DISTRICT OF INDIANA INDIANAPOLIS DIVISION.
- https://nida.nih.gov/publications/drugfacts/synthetic-cannabinoids-k2spice
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5999798/
- https://publications.aap.org/aapnews/news/14002/Study-Seizures-coma-more-common-with-synthetic
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9875316
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4826922/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3863350/
- https://pubs.acs.org/doi/10.1021/acs.chemrestox.2c00170
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9033635/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939204/
- https://pubmed.ncbi.nlm.nih.gov/31389266/
- https://downloads.usda.library.cornell.edu/usda-esmis/files/gf06h2430/76538f824/w9506f61g/hempan23.pdf
- https://projectcbd.org/hemp/expert-gives-delta-8-thc-a-thumbs-down
- https://www2.texasattorneygeneral.gov/files/initiatives/synth/Synthetic_drug_factsheet_accessible.pdf
- https://cen.acs.org/biological-chemistry/natural-products/Delta-8-THC-craze-concerns/99/i31
- https://projectcbd.org/hemp/expert-gives-delta-8-thc-a-thumbs-down/
- https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00197-6
- https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00197-6
- https://shop.cookies.co/pages/shipping-policy
- https://deltadreamz.com/shop/ols/products/cap-n-chronic-1000mg
- https://www.kratomstoresohio.com/delta-8-products/
- https://dazeddragons.com/store/delta-8-jolly-rancher/
- https://www.cbdsupplymd.com/shop/skittles-wild-berry-delta-8-400mg-20-count/
- https://publications.aap.org/pediatrics/article/151/2/e2022057761/190427/Pediatric-Edible-Cannabis-Exposures-and-Acute
- https://www.cdc.gov/mmwr/volumes/72/wr/mm7228a1.htm?s_cid=mm7228a1_w
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c018be7d-f7b8-45e2-97b8-8e7a71740657
- https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00197-6
- https://weedmaps.com/learn/products-and-how-to-consume/decide-how-much-to-take
- Including Maryland and Montana.
- https://sensiseeds.com/en/blog/can-cbd-counteract-the-effects-of-thc/
- https://doi.org/10.1038/s41386-022-01478-z
- https://pubmed.ncbi.nlm.nih.gov/36380220/
- https://pubmed.ncbi.nlm.nih.gov/30661105/
- https://www.conflabs.com/why-0-877/
- https://cannabusiness.law/thca-flower-the-next-big-thing-in-hempland
- https://www.law.cornell.edu/uscode/text/21/813
- See AK Futures, LLC v. Boyd Street Distro, LLC, 35 F4th 682 (9th. Cir. 2022)
- https://www.courthousenews.com/wp-content/uploads/2023/06/Indiana-hemp-AG-opinion-lawsuit.pdf
- https://cannabislaw.report/lawsuit-filed-in-georgia-argues-delta-8-and-delta-10-products-are-legal-under-state-law/
- https://mjbizdaily.com/wp-content/uploads/2022/05/HF3595.2.pdf
- https://pubmed.ncbi.nlm.nih.gov/36264171/
- https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00197-6